
 |
|
|||||||
| Forum Rules | Firearms Safety | Firearms Photos | Links | Library | Lost Password | Email Changes |
| Register | FAQ | Calendar | Today's Posts | Search |
 |
|
|
Thread Tools | Search this Thread |
|
|
#26 | ||||
|
Senior Member
Join Date: May 27, 2007
Posts: 5,261
|
Quote:
As nitric acid gas comes out of the powder it causes rust. Rust is an ionic compound and all ionic compounds interact with the double bonds on nitrocellulose , breaking them, and releasing NOx. Nitroglycerine also interacts with those double bonds which is why double based powder have a lifetime less than half of single based. Water is bad for several reasons. I found a paper in the 30’s where water dissolved some of the gelatinizing compounds in gunpowder and caused micro cracking of the grains. Water is a polar covalent ion and acts ionic, the big oxygen atom at the end interacts with double bonds and breaks them. Water in the air also has the unfortunate effect of condensing and evaporating on the powder grain, and when water evaporates it wicks nitroglycerine to the surface. I met a gentleman who had been to a small rocket motor recertification facility. These were old, Korean war or earlier, rocket motors used for sled track purposes. He saw the workers scrape off nitroglycerine off the surface of the opened motors. While these motor were old, they were are unique in their size and availability. They also periodically blow in tests. While unfortunate, these are not manned tests, and the tradeoff is, no test, or a test with a probability of rocket failure. At some point, obviously, the rockets get dumped, because no one wants to fund a test with a 50-50 chance of failure. They don't want to fund a test with a 5% chance of failure either. When the surface of any powder becomes nitrogylercine rich, that will spike the initial pressure curve. So while it does not make sense, you would expect that gunpowder as it breaks down, would loose energy, and thus go benign, and the last part will eventually happen, but even as it is losing energy, it is changing its burn rate characteristics. That is what spikes the pressure curve. Mechanical deterioration, which you see in the pictures, that dust which results, is very bad. Increasing the surface area will vastly spike the pressure curve. You would think coal is low energy compared to gunpowder, and it more or less is, but grind it into a dust, and a coal dust explosion is real impressive, and dangerous. Coal dust explosion in container http://www.youtube.com/watch?v=8Cdhm024-10 Coal Dust explosion, industrial level: http://www.liveleak.com/view?i=09e_1241559869 Suppressed versus unsuppressed coal dust explosion. http://www.youtube.com/watch?v=czUUlJQ_9LE http://www.mineweb.com/mineweb/conte...7297&sn=Detail Quote:
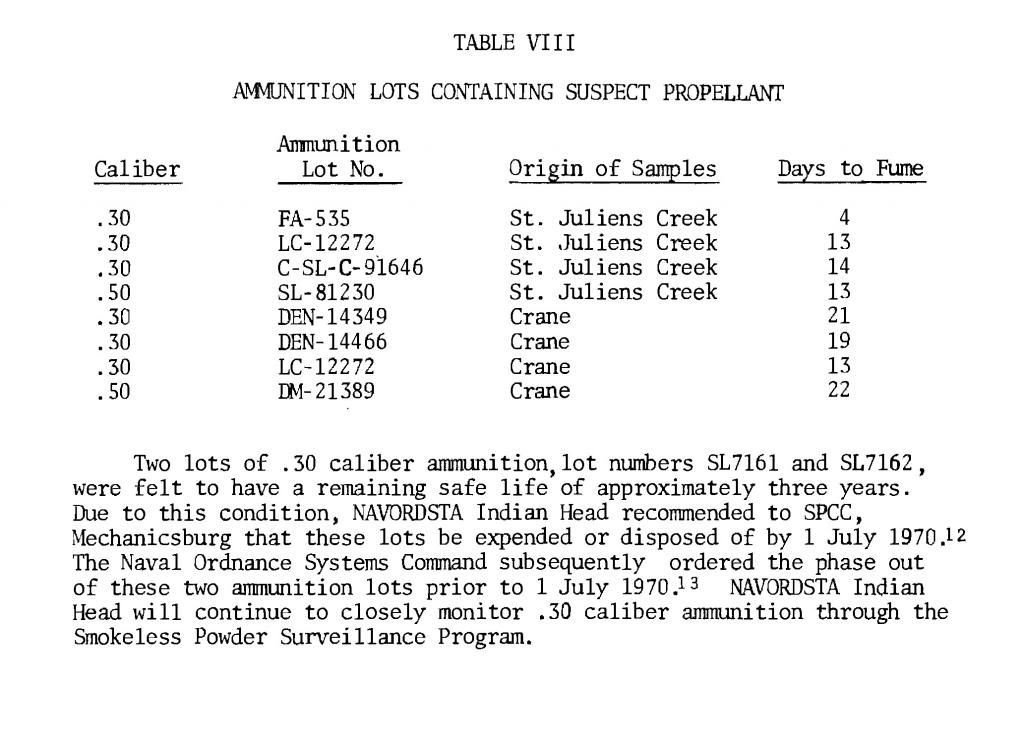
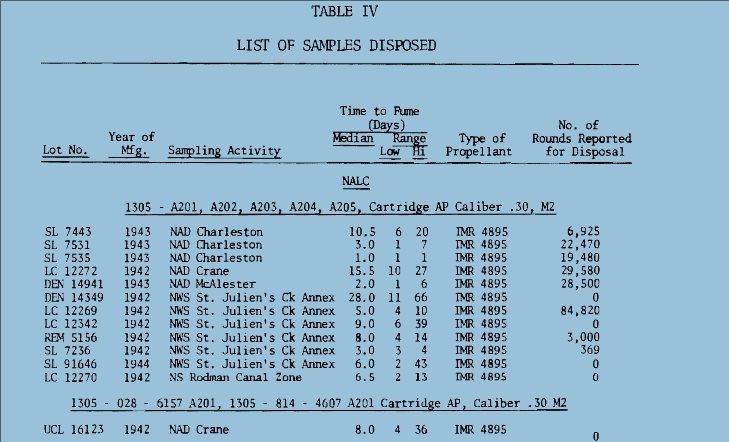
 There is not enough powder in a single case to smell anything. Surplus IMR powder that did not have a smell in the keg, still black in color, went bad in a couple of years, I had corrosion, case neck splits, pressure indications. I am now suspicious of any powders that are neutral in smell. There is not enough powder in a single case to smell anything. Surplus IMR powder that did not have a smell in the keg, still black in color, went bad in a couple of years, I had corrosion, case neck splits, pressure indications. I am now suspicious of any powders that are neutral in smell. All I really want to do is inform the community that gunpowder does not last forever, understand the mechanisms, to the best of my understanding, of how gunpowder breaks down, what to look for, and to understand that old ammunition has its risks. I would recommend to all, if you experience pressure indications with old ammunition, if you see corrosion, stop shooting the ammunition, dump the powder, save the bullets. I would inspect the cases for evidence of corrosion, and then I would ponder what to do with the cases. No corrosion would be a good thing, and as I am cheap, I might reuse them. I might be spooked by another’s experience and toss them all out. I could go either way. Quote:
__________________
If I'm not shooting, I'm reloading. Last edited by Slamfire; March 2, 2014 at 02:05 PM. |
||||
|
|
|
|
#27 |
|
Senior Member
Join Date: August 30, 2010
Posts: 1,635
|
Interesting - I was just reading today that when the USS Iowa's main battery exploded back in the 80's or 90's or whenever it was it is though that the culprit may have been powder manufactured in the 1930s.
Steve |
|
|
 |
|
|