
 |
|
|||||||
| Forum Rules | Firearms Safety | Firearms Photos | Links | Library | Lost Password | Email Changes |
| Register | FAQ | Calendar | Today's Posts | Search |
 |
|
|
Thread Tools | Search this Thread |
|
|
#1 |
|
Senior Member
Join Date: December 31, 2011
Location: Vermont
Posts: 2,076
|
Corrosion Like I Have Never Seen
I inherited a bit of ammo when my Father died, and among that cache was a box of Norma 220gr .30-06 (from the 60's by the look of the box)...
I pulled them out to look at them this AM, and this is what I found:  Five out of 16 rounds have this corrosion on them...On two, the corrosion has expanded the case below the bullet to the extent where the brass ruptured...This box was stored bullet down for decades at Dad's, and for a few years now here...They had a cardboard divider in the box so none touched, and there is no sign of water damage to the box or divider...I have looked through the rest of the 06 ammo boxes, and all are pristine like they came from the factories yesterday (but none are Norma)... What the heck am I seeing here? Is this corrosive primers gone bad and dripped through the powder to the base of the bullet and then eaten through, or is this deteriorating powder fuming through the case? I obviously am not going to fire any of these rounds, but are they even safe to subject to bullet pulling in an impact puller??? I'd love to salvage the bullets at least... Any help or opinions would be appreciated... |
|
|
|
|
#2 |
|
Senior Member
Join Date: November 6, 2001
Posts: 1,131
|
Powder looks to have gone bad and created acetic acid which has eaten through the case. If you pull the bullets you'll probably find the bullet base has been corroded too and probably fused to the case. Worth a try to salvage the bullets but I bet they are in bad shape? Wash your hands after handling those components. Worse corrosion of old ammo that I've seen.
|
|
|
|
|
#3 |
|
Staff
Join Date: April 14, 2000
Location: Northern Virginia
Posts: 41,642
|
Oh yeah, bad powder. VERY bad powder.
Don't attempt to shoot the ones that "look" good. Ditch it ALL.
__________________
"The gift which I am sending you is called a dog, and is in fact the most precious and valuable possession of mankind" -Theodorus Gaza Baby Jesus cries when the fat redneck doesn't have military-grade firepower. |
|
|
|
|
#4 |
|
Senior Member
Join Date: August 26, 2008
Location: In the valley above the plain
Posts: 13,774
|
As Mike said....
Bad powder. Get rid of it all.
__________________
-Unwilling Range Officer -Unwilling Match Designer -NRL22/PRS22/PRO -Something about broccoli and carrots |
|
|
|
|
#5 |
|
Senior Member
Join Date: December 31, 2011
Location: Vermont
Posts: 2,076
|
Thanks guys...
Had no intention of shooting any of them once I found the first one looking like that...Was more concerned about having one blast off if this was a primer compound sensitivity issue... Broke down the ones I could... Every one had brown dust to one degree, and verdigris in the case:  This is one of the lesser evils:  This is one of the ones that had ruptured the case:  Having fired cartridges and shot-shells from well before WWII with no issues at all, I have always been a great proponent of old ammo... I can safely say this experience is making me call my laissez-faire attitude towards old ammo into question... Even the cartridges with absolutely no outward signs of issues may have been dangerous... |
|
|
|
|
#6 |
|
Senior Member
Join Date: December 14, 2004
Location: Northern Indiana
Posts: 6,117
|
WOW!
I picked up 400+ rounds in M1 Grand in end blocks of 1942 30-06 for pennies a round several years ago. I pulled all the bullets and primers. None of them had any corrosion and the powder looked perfect. I ran the cases in a vibrating case cleaner and the inside looked clean and shiny. I find it strange that rounds like that would turn into that junk from the 60’s I have several rounds of loaded 1856 brass Burnsides and although I won’t pull the bullets the outside of the case looks shiny. Man that is strange. Question to the experts, how does powder turn bad like that? |
|
|
|
|
#7 | |
|
Senior Member
Join Date: December 31, 2011
Location: Vermont
Posts: 2,076
|
Quote:
|
|
|
|
|
|
#8 |
|
Member
Join Date: April 30, 2009
Location: San Antonio, TX
Posts: 99
|
I inherited some loaded in the 30s that did the same thing. My guess is that the loading and storage conditions were less than ideal. Hand loading without air-conditioning in hot, humid weather and then storing without climate control should accelerate the powder breakdown.
|
|
|
|
|
#9 |
|
Senior Member
Join Date: September 28, 2008
Posts: 10,442
|
Mice, definitely mice.
Those little rascals will eat anything. |
|
|
|
|
#10 |
|
Senior Member
Join Date: December 31, 2011
Location: Vermont
Posts: 2,076
|
For pete's sake...
I just put a magnet to the bullets, and they are steel jacketed that is apparently copper washed... I got the idea from looking at the base of the bullets I pulled, and noticed that boat-tail seemed to be a different metal...The boat-tail seems to be a cap like a gas check, and does not look like it was plated... Could steel have caused a reaction with the powder? I know that I had old steel powder cans that were 'tinned' on the inside... |
|
|
|
|
#11 |
|
Senior Member
Join Date: February 26, 2013
Posts: 249
|
now why did you have to go and take them apart. they were just alittle dirty and would have been safe to shoot

|
|
|
|
|
#12 |
|
Senior Member
Join Date: January 3, 2008
Location: Central Oregon
Posts: 348
|
Had to be a reaction to the bullet . All of the corrosion is at the base of the bullet . The only propellant I have ever seen go really bad was the cordite loaded stuff the Britts used in WWII . It would be nasty down the entire length of the case .
|
|
|
|
|
#13 |
|
Staff
Join Date: April 14, 2000
Location: Northern Virginia
Posts: 41,642
|
"Question to the experts, how does powder turn bad like that?"
Nitrocellulose gunpowder is an inherently unstable chemical compound. It has to be, or otherwise it wouldn't work as gunpowder. Nitrocellulose powder naturally wants to decay. To increase the shelf, chemicals are added, which as a class are called stabilizers. Over time these stabilizers become used up, meaning that the powder is now actively decaying. As powder decays, one of the byproducts is a very strong acidic residue which attacks the case, and is what caused what you saw in the Norma ammo. Depending on storage conditions, nitrocellulose powder can remain viable for many years, but it's not as simple or as safe as some of us have come to believe. I had always thought that when powder decayed with time it became less potent. That's not the case. Member Slamfire has posted a wealth of primary source information that indicates that decaying powder can become less powerful, or it can become significantly more powerful, and it's something of a luck of the draw at what point the powder is in its decay cycle. "Could steel have caused a reaction with the powder?" No. The steel reacted to corrosive elements released by the decaying powder. The reason why the brass failed at the neck? Well, as you indicated, the ammo may have been stored bullet down for along time... That MIGHT have had something to do with it, but I sort of doubt it... But the realistic reason? The brass is thinnest at the shoulder and base of the neck. It's going to eat through there first.
__________________
"The gift which I am sending you is called a dog, and is in fact the most precious and valuable possession of mankind" -Theodorus Gaza Baby Jesus cries when the fat redneck doesn't have military-grade firepower. Last edited by Mike Irwin; February 28, 2014 at 11:14 AM. |
|
|
|
|
#14 | ||
|
Senior Member
Join Date: May 27, 2007
Posts: 5,261
|
Samoneye: Great pictures! Can I use them when discussing this topic?
As Mike stated, gunpowder has a shelf life. Our gunpowders are either nitrocellulose or nitrocellulose & nitroglycerine. The nitroglycerine is there basically for the energy boast. Because nitroglycerine attacks the double bonds on nitrocellulose the lifetime of double based powders is less than half that of single based. Stabilizers are mixed with the nitrocellulose/nitroglycerine as a sacrificial compound: stabilizers soak up the nitric acid gas that is created when nitrocellulose deteriorates. Without a doubt, this is the reason for the corrosion in your pictures. When the stabilizer gets low , gunpowder is extremely unstable and unsafe. In quantity it will auto combust and the burn rate is irregular. Burn rate instability has and will blow up firearms. A good rule of thumb is that single base powders will last 45 years and double based 20 years. Like all rules of thumb this is wrong more often than it is right. Federal says their ammunition has a ten year shelf life: Federal Ammunition : http://www.federalpremium.com/company/faq.aspx Quote:
This thread has some really excellent pictures of 25 year old powder that went bad in the case: Has anyone else had Vihtavuori N140 corrode in loaded ammo? http://www.falfiles.com/forums/showthread.php?p=3745264 It was not that long ago that 20 years was considered the shelf life of gunpowder. Army Ordnance Magazine, May 1931, Safety Hazards, Picatinny Arsenal Smokeless powder constitutes one of the greatest hazards from a storage standpoint, due to the fact that is subject to deterioration and at the best cannot be expected to have a life greater than about twenty years The lifetime of gunpowder is reduced exponentially with increases in temperature. This chart came from a UN manual on ammunition inspection. Section 7.3 is well worth reading Surveillance and in-service proof - the United Nations http://www.un.org/disarmament/convar...Proof(V.1).pdf [URL=http://smg.photobucket.com/user/SlamFire/media/Reloading/Old%20Gunpowder/Propellantdeteriorationyearsversustemperature_zps2 9357560.png.html] [/URL There is very little information on the internet about gunpowder aging and the pressure problems this will create, because all that was ever needed to be known was determined well before WW2, well before the electronic age. I have gone to the library and researched bound volumes of the Army Ordnance Magazine from the 20's up to WW2 and found information, but hardly anyone does this anymore. The pre electronic literature is a black hole in this internet age. Ball powders did come out at the end of WW2 and I was able to find this data showing that gunpowder at the end of its lifetime will pressure spike. Heat is used to accelerate the age of gunpowder, so what you are seeing is in fact because of “age”, not heat, but it took heat to age the powder quickly. The IMR is a single based and the WC is a double based ball powder. INVESTIGATION OF THE BALLISTIC AND CHEMICAL STABILITY OF 7.62MM AMMUNITION LOADED WITH BALL AND IMR PROPELLANT Frankfort Arsenal 1962 3. Effects of Accelerated Storage Propellant and Primer Performance To determine the effect of accelerated isothermal storage upon propellant and primer performance, sixty cartridges from each of lots E (WC 846) and G (R 1475) were removed from 150F storage after 26 and 42 weeks, respectively. The bullets were then removed from half the cartridges of each lot and from an equal number of each lot previously stored at 70F. The propellants were then interchanged, the bullets re-inserted, and the cases recrimped. Thus, four variations of stored components were obtained with each lot. Chamber pressures yielded by ammunition incorporating these four variations were as follows. These values represent averages of 20 firings. 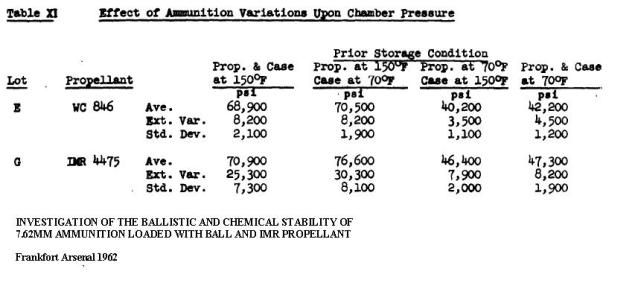 You can google “surplus ammunition Kaboom” or the equivalent and find a surprising number of reports of firearms that have blown up with old military ammunition. Now I am going to tell you the terrible truth about surplus ammunition. The stuff was surplused because it was at the end of its service life and the Army that owned it, determined that it was unsafe to store and unsafe to shoot. Some Ammunition Specialist went through that lot and found evidence that the gunpowder had deteriorated and the stuff was sold to eager Americans who do not know about the risks of old ammunition. Why some powder goes bad quickly and other lasts longer, heck if I know. The strangeness of this can be found in this thread at Post 61. This is our own Unclenick’s experience and it is worth looking at the picture: Old can of 2400 http://thefiringline.com/forums/show...6&postcount=61 Quote:
__________________
If I'm not shooting, I'm reloading. Last edited by Slamfire; March 2, 2014 at 12:54 PM. |
||
|
|
|
|
#15 | |
|
Senior Member
Join Date: December 31, 2011
Location: Vermont
Posts: 2,076
|
Quote:
Right click the images and choose 'Save As'. or 'Save Image' and save them to your hard drive so you will have them in the future... I thought of you when I found these rounds...I have read your posts on powder life, and I must admit was one that 'scoffed'... I am seriously rethinking my position at this point... Thanks for the thread link...Great pics... |
|
|
|
|
|
#16 |
|
Senior Member
Join Date: November 19, 2009
Posts: 3,290
|
Very interesting post and thanks for sharing those pictures. That's some nasty stuff!
I used to have a good assortment of rifle ammo that had been given to me by folks who had it, didn't know what to do with it and knew I liked guns. Most of it was in boxes such as described - stored bullet down with card board dividers, etc. I ended up giving it away as I didn't shoot the calibers but I don't believe I ever saw anything as bad as that. Thanks for sharing and all of the responses as to why it ended up that way.
__________________
If a pair of '51 Navies were good enough for Billy Hickok, then a single Navy on my right hip is good enough for me . . . besides . . . I'm probably only half as good as he was anyways. Hiram's Rangers Badge #63 |
|
|
|
|
#17 | ||||
|
Senior Member
Join Date: May 27, 2007
Posts: 5,261
|
Quote:
 Quote:
Quote:

__________________
If I'm not shooting, I'm reloading. |
||||
|
|
|
|
#18 |
|
Senior Member
Join Date: November 30, 2012
Location: Spring City, PA
Posts: 497
|
Dont forget about triple based powders! And believe it or not, the powder you have on your shelf that you just bought the other day is already undergoing a constant chemical reaction. Pretty neat stuff though with the stabilizers. My fiancee is a biochemist and she just informed me that cellulose simply means fiberous. So the stabilizers that are used are like micro sponges that absorb the nitric acid that is constantly leached. Once the "sponges" are no longer to absorb the deteriorating byproducts, the chemical reaction speeds up and causes heat. Heat is a natural occurance in anything that is decaying. And given that everything has a "flash point", once the chemical reaction produces enough heat to reach theflash point....boom. this can also be seen in corn silos and.compost piles.
|
|
|
|
|
#19 | |
|
Senior Member
Join Date: May 27, 2007
Posts: 5,261
|
I don't know why I have not found any reference to triple based powders and small arms, but I have no doubt they are all breaking down.
I was told that stabilizers have not changed much since the 1880's! This has a list of common stabilizers: http://www.islandgroup.com/military/...tabilizers.php This DTIC document has a bit of history on diphenylamine: ROLE OF DIPHENYLAMINE AS A STABILIZER IN PROPELLANTS; ANALYTICAL CHEMISTRY OF DIPHENYLAMINE IN PROPELLANTS Quote:
This section from the Propellant Management Guide explains it all: Stabilizers are chemical ingredients added to propellant at time of manufacture to decrease the rate of propellant degradation and reduce the probability of auto ignition during its expected useful life. As nitrocellulose-based propellants decompose, they release nitrogen oxides. If the nitrogen oxides are left free to react in the propellant, they can react with the nitrate ester, causing further decomposition and additional release of nitrogen oxides. The reaction between the nitrate ester and the nitrogen oxides is exothermic (i.e., the reaction produces heat). Heat increases the rate of propellant decomposition. More importantly, the exothermic nature of the reaction creates a problem if sufficient heat is generated to initiate combustion. Chemical additives, referred to as stabilizers, are added to propellant formulations to react with free nitrogen oxides to prevent their attack on the nitrate esters in the propellant. The stabilizers are scavengers that act rather like sponges, and once they become “saturated” they are no longer able to remove nitrogen oxides from the propellant. Self-heating of the propellant can occur unabated at the “saturation” point without the ameliorating effect of the stabilizer. Once begun, the self-heating may become sufficient to cause auto ignition. It would be more accurate, as the guide states, to say that NOx is released. NOx is a series of compounds but nitric acid gas is one, if there is water around. But who wants to read this stuff anyway?, I probably put most people to sleep with what I have written. Nitrocellulose was made from cotton, I guess that is where the cellulose part of the name came from.
__________________
If I'm not shooting, I'm reloading. |
|
|
|
|
|
#20 |
|
Staff
Join Date: April 14, 2000
Location: Northern Virginia
Posts: 41,642
|
Triple base propellants are generally used for artillery and large guns. I don't believe that they have been used much in small arms.
__________________
"The gift which I am sending you is called a dog, and is in fact the most precious and valuable possession of mankind" -Theodorus Gaza Baby Jesus cries when the fat redneck doesn't have military-grade firepower. |
|
|
|
|
#21 |
|
Senior Member
Join Date: November 13, 2002
Location: Red River Valley of the North
Posts: 203
|
It might help some people to just google gunpowder shelf life and do a little reading. I don't doubt slamfires comments or experiences - his experiences are just different from mine and all the shooters that I have known. I also have no idea why some commerical ammo goes to hell in a hand basket.
One example I'll mention is Hodgdon powder, namely the H4831 was 1st on the market back in the 1950’s and to my understanding was actually military surplus powder made back in the 1930’s. There are some people still shooting that “original” powder and it is performing as good today as it did back in the 1950’s. I think Hodgdon ran out of that particular military surplus powder back in the ‘70’s and thereafter labeled their containers “newly manufactured” and that powder was sold in the ole paper container colored red & black with a black plastic cap. That "newly manufactured" powder is still going strong today. I have never come across any smokeless gun powder that smelled “good” regardless of age, that didn’t perform as expected. In all my years of reloading, I’ve only come across one case of IMR-4350 in the brown metal can with red cap having white & brown lettering that went bad while stored in ideal conditions for about 20 years. When given the sniff test it failed badly!! I suspect it was the containers that broke the powder down, as I had some of that powder from that particular case stored in my "plastic pharmaceutical pill reloading bottle" that never went bad and it's is still good today. In my opinion, most smokeless gun powder will outlive the user if stored in “reasonable” conditions over the years and all that is needed to determine if the powder is good or not is your sense of smell. Last edited by Ole 5 hole group; March 2, 2014 at 07:52 AM. |
|
|
|
|
#22 | |
|
Senior Member
Join Date: August 30, 2009
Location: Northern AZ
Posts: 7,172
|
Quote:
|
|
|
|
|
|
#23 | |
|
Senior Member
Join Date: December 31, 2011
Location: Vermont
Posts: 2,076
|
Quote:
Some of the cartridges I disassembled had no outward appearance of problems, but internally were a different story... And there is no way to give them a smell test... |
|
|
|
|
|
#24 |
|
Senior Member
Join Date: December 11, 2012
Posts: 527
|
This is a GREAT thread and explains why my buddies old rifle in 30-06 blew up on him when we were shooting his granddads old ammo. We shot boxes of the stuff with no issues and these rounds didnt look any different from the others.
Lesson learned then and 20+ years later I know why it happened. |
|
|
|
|
#25 |
|
Staff
Join Date: April 14, 2000
Location: Northern Virginia
Posts: 41,642
|
Yes, Hodgdon got its start selling surplus powder.
It wasn't from the 1930s, though, it was military production surplus from World War II. And I have seen batches of that powder that has gone bad. There's simply no realistic test that can tell us just how viable older powder is. The military may have such tests, but I suspect that they are beyond any of us, unless we have a full chemical engineering lab at our disposal. There's a good reason why the military surpluses ammo after a certain number of years -- because from their own research and experience, they KNOW that powder degrades over time. I used to think of powder as being an ageless thing unless it was stored in absolutely abysmal conditions. It's not. I have hundreds of rounds of ammunition in my personal collection, many of them from the dawn of the smokeless age. They all seem to be viable. I've even cracked some old (really old) cartridges. The powder SEEMS to be OK, but again, without that lab set up, I have no clue what's going on with that powder at a molecular level, nor am I going to make any bets. I'll probably even keep shooting old surplus ammo from time to time, but I'll be a lot more circumspect about it than I used to be.
__________________
"The gift which I am sending you is called a dog, and is in fact the most precious and valuable possession of mankind" -Theodorus Gaza Baby Jesus cries when the fat redneck doesn't have military-grade firepower. |
|
|
 |
|
|